- Your Product Type
- Your Study Type
- Aquatic Ecotoxicology
- Aquatic Invertebrates
- OECD 202: Daphnia sp., Acute Immobilisation Test
- OECD 211: Daphnia magna Reproduction Test
- OECD 235: Chironomus sp., Acute Immobilisation Test
- OECD 218/219: Sediment-Water Chironomid Toxicity Test Using Spiked Sediment/Spiked Water
- OECD 233: Sediment-Water Chironomid Life-Cycle Toxicity Test Using Spiked Water or Spiked Sediment
- OECD 225: Sediment-water Lumbriculus Toxicity Test Using Spiked Sediment
- OECD 242: Potamopyrgus antipodarum Reproduction Test
- OECD 243: Lymnaea stagnalis Reproduction Test
- Fish and other vertebrates
- OECD 203: Fish, Acute Toxicity Test
- OECD 215: Fish Juvenile Growth Study
- OECD 212: Fish, Short-term Toxicity Test on Embryo and Sac-fry Stages
- OECD 231: The Amphibian Metamorphosis Assay
- OECD 236: Fish Embryo Acute Toxicity Test
- OECD 210: Fish, Early-life Stage Toxicity Test
- OECD 229 Fish Short Term Reproduction Assay and OECD 230 21-day Fish Assay
- OECD 240 Medaka Extended One Generation Reproduction Test (MEOGRT)
- OECD 248: Xenopus Eleutheroembryonic Thyroid Assay
- OPPTS 850.1500: Fish Life Cycle Toxicity Test
- OÈCD 234 Fish sexual development test
- Aquatic plants
- Analytical Dose Verification
- Aquatic Invertebrates
- Chemistry
- Biodegradation Studies
- Analytical Chemistry Studies and Residues
- Physical-Chemical Properties Studies
- Storage Stability Studies
- OPPTS 830.6302, OPPTS 830.6303,and OPPTS 830.6304: Physical State, Colour and Odor at 20 °C and at 101.3 kPa
- EU A.1: Melting temperature/range
- EU A.2: Boiling temperature
- EU A.3: Relative density (liquids and solids)
- EU A.4: Vapour pressure
- EU A.5: Surface tension
- EU A.9: Flashpoint
- EU A.10: Flammability (solids)
- EU A.12: Flammability (contact with water)
- EU A.13: Pyrophoric properties of solids and liquids
- EU A.16: Relative self-ignition temperature for solids
- EU A.17: Oxidising properties
- OECD 114: Viscosity of Liquids
- Environmental Fate
- Transformation in Soil
- Transformation in Water
- Transformation in Manure
- Adsorption on Soil and Sewage Sludge
- Bioaccumulation and Bioconcentration
- Terrestrial Ecotoxicology
- Non-target Arthropods
- Non-target arthropod testing with the parasitic wasp (Aphidius rhopalosiphi)
- Non-target arthropod testing with the lacewing (Chrysoperla carnea)
- Non-target arthropod testing with the ladybird beetle (Coccinella septempunctata)
- Non-target arthropod testing with the predatory bug (Orius laevigatus)
- Non-target arthropod testing with the predatory mite (Typhlodromus pyri)
- Non-target arthropod testing with the rove beetle (Aleochara bilineata)
- Non-target arthropod testing with the carabid beetle (Poecilus cupreus)
- Non-target arthropod testing with the wolf spider (Pardosa spec.)
- Soil Organisms
- Honey Bees and other Pollinators
- OECD 213/214: Honey bees, Acute Oral and Acute Contact Toxicity Test
- OECD 245: Honey Bee (Apis Mellifera L.), Chronic Oral Toxicity Test (10-Day Feeding)
- OECD 237: Honey Bee Larval Toxicity Test, Single Exposure
- OECD 239: Honey Bee Larval Toxicity Test
- EPPO 170: Honey Bee Field Study – do plant protection products effect honey bee colonies?
- Oomen et al. 1992: Honey Bee Brood Feeding Study
- OECD 75: Honey Bee Brood Test under Semi-field Conditions in Tunnels
- OECD 246/247 Acute Oral and Contact Toxicity to the Bumblebee, Bombus terrestris L.
- Solitary Bee Acute Contact Toxicity Study in the Laboratory (Osmia sp.) Solitary Bee Acute Oral Toxicity Study in the Laboratory (Osmia sp.) (protocols for ringtests with solitary bees recommended by the non-Apis working group)
- SANTE/11956/2016 rev.9 Residue trials for MRL setting in honey
- Non-target plants
- OECD 208: Terrestrial Plant Test - Seedling Emergence and Seedling Growth Test
- OECD 227: Terrestrial Plant Test - Vegetative Vigour Test
- OCSPP 850.4100: Seedling Emergence and Seedling Growth
- OCSPP 850.4150: Vegetative Vigor
- EPPO PP 1/207(2): Efficacy evaluation of plant protection products, Effects on succeeding crops
- Field Studies
- Non-target Arthropods
- Ecological Modelling
- Quality Assurance
- Testing of Potential Endocrine Disruptors
- Aquatic Ecotoxicology
- News
- Company
- Career
- Contact
OECD 215: Fish Juvenile Growth Study
Protection of the fish community in freshwater is an important issue because fish play a major role in trophic cascades and as food resource for human beings. Therefore the impact on fishes of plant protection products, industrial chemicals, biocides and medicinal products that may get into aquatic environment has to be assessed.
Study Design
Test organisms
- Oncorhynchus mykiss (rainbow trout)
- Danio rerio (zebrafish)
- Oryzias latipes (Japanese medaka)

 Course of the test
Course of the test
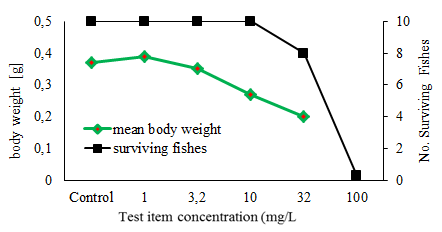
The Fish Juvenile Growth study is designed to assess the effects of prolonged exposure to chemicals on the growth of juvenile fish.
Juvenile fish in exponential growth phase are placed, after being weighed, in test chambers and are exposed to a range of sublethal concentrations of the test substance dissolved in water preferably under flow-through or semi-static conditions for 28 days. Usually 5 concentrations in a geometric row and a control are tested. Results of an acute toxicity test, preferably performed with the species chosen for this test, should be available. The fish are fed daily, with a food ration based on initial fish weights. At the end of the test, the fish are weighed again.
Endpoints
During the test period the fish are observed daily for survival, abnormal appearance and behaviour. Additionally, at the end of the test the weigth of all surviving fish is determined. The specific growth rate is calculated and the concentraion that would cause a x % variation in growth rate, i.e. ECx (e.g. EC10, EC20 or EC30) is analysed. The lowest observed effect concentration (LOEC) and the no observed effect concentration (NOEC) are estimated.
Guidelines and literature
- OECD Guideline for Testing of Chemicals, No. 215: “Fish, Juvenile Growth Test“, adopted January 21, 2000
