- Your Product Type
- Your Study Type
- Aquatic Ecotoxicology
- Aquatic Invertebrates
- OECD 202: Daphnia sp., Acute Immobilisation Test
- OECD 211: Daphnia magna Reproduction Test
- OECD 235: Chironomus sp., Acute Immobilisation Test
- OECD 218/219: Sediment-Water Chironomid Toxicity Test Using Spiked Sediment/Spiked Water
- OECD 233: Sediment-Water Chironomid Life-Cycle Toxicity Test Using Spiked Water or Spiked Sediment
- OECD 225: Sediment-water Lumbriculus Toxicity Test Using Spiked Sediment
- OECD 242: Potamopyrgus antipodarum Reproduction Test
- OECD 243: Lymnaea stagnalis Reproduction Test
- Fish and other vertebrates
- OECD 203: Fish, Acute Toxicity Test
- OECD 215: Fish Juvenile Growth Study
- OECD 212: Fish, Short-term Toxicity Test on Embryo and Sac-fry Stages
- OECD 231: The Amphibian Metamorphosis Assay
- OECD 236: Fish Embryo Acute Toxicity Test
- OECD 210: Fish, Early-life Stage Toxicity Test
- OECD 229 Fish Short Term Reproduction Assay and OECD 230 21-day Fish Assay
- OECD 240 Medaka Extended One Generation Reproduction Test (MEOGRT)
- OECD 248: Xenopus Eleutheroembryonic Thyroid Assay
- OPPTS 850.1500: Fish Life Cycle Toxicity Test
- OÈCD 234 Fish sexual development test
- Aquatic plants
- Analytical Dose Verification
- Aquatic Invertebrates
- Chemistry
- Biodegradation Studies
- Analytical Chemistry Studies and Residues
- Physical-Chemical Properties Studies
- Storage Stability Studies
- OPPTS 830.6302, OPPTS 830.6303,and OPPTS 830.6304: Physical State, Colour and Odor at 20 °C and at 101.3 kPa
- EU A.1: Melting temperature/range
- EU A.2: Boiling temperature
- EU A.3: Relative density (liquids and solids)
- EU A.4: Vapour pressure
- EU A.5: Surface tension
- EU A.9: Flashpoint
- EU A.10: Flammability (solids)
- EU A.12: Flammability (contact with water)
- EU A.13: Pyrophoric properties of solids and liquids
- EU A.16: Relative self-ignition temperature for solids
- EU A.17: Oxidising properties
- OECD 114: Viscosity of Liquids
- Environmental Fate
- Transformation in Soil
- Transformation in Water
- Transformation in Manure
- Adsorption on Soil and Sewage Sludge
- Bioaccumulation and Bioconcentration
- Terrestrial Ecotoxicology
- Non-target Arthropods
- Non-target arthropod testing with the parasitic wasp (Aphidius rhopalosiphi)
- Non-target arthropod testing with the lacewing (Chrysoperla carnea)
- Non-target arthropod testing with the ladybird beetle (Coccinella septempunctata)
- Non-target arthropod testing with the predatory bug (Orius laevigatus)
- Non-target arthropod testing with the predatory mite (Typhlodromus pyri)
- Non-target arthropod testing with the rove beetle (Aleochara bilineata)
- Non-target arthropod testing with the carabid beetle (Poecilus cupreus)
- Non-target arthropod testing with the wolf spider (Pardosa spec.)
- Soil Organisms
- Honey Bees and other Pollinators
- OECD 213/214: Honey bees, Acute Oral and Acute Contact Toxicity Test
- OECD 245: Honey Bee (Apis Mellifera L.), Chronic Oral Toxicity Test (10-Day Feeding)
- OECD 237: Honey Bee Larval Toxicity Test, Single Exposure
- OECD 239: Honey Bee Larval Toxicity Test
- EPPO 170: Honey Bee Field Study – do plant protection products effect honey bee colonies?
- Oomen et al. 1992: Honey Bee Brood Feeding Study
- OECD 75: Honey Bee Brood Test under Semi-field Conditions in Tunnels
- OECD 246/247 Acute Oral and Contact Toxicity to the Bumblebee, Bombus terrestris L.
- Solitary Bee Acute Contact Toxicity Study in the Laboratory (Osmia sp.) Solitary Bee Acute Oral Toxicity Study in the Laboratory (Osmia sp.) (protocols for ringtests with solitary bees recommended by the non-Apis working group)
- SANTE/11956/2016 rev.9 Residue trials for MRL setting in honey
- Non-target plants
- OECD 208: Terrestrial Plant Test - Seedling Emergence and Seedling Growth Test
- OECD 227: Terrestrial Plant Test - Vegetative Vigour Test
- OCSPP 850.4100: Seedling Emergence and Seedling Growth
- OCSPP 850.4150: Vegetative Vigor
- EPPO PP 1/207(2): Efficacy evaluation of plant protection products, Effects on succeeding crops
- Field Studies
- Non-target Arthropods
- Ecological Modelling
- Quality Assurance
- Testing of Potential Endocrine Disruptors
- Aquatic Ecotoxicology
- News
- Company
- Career
- Contact
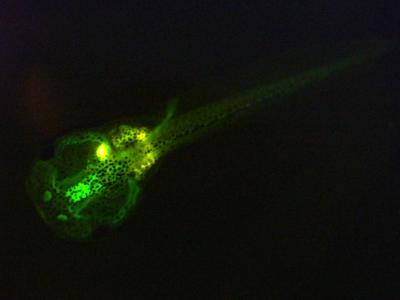
OECD 248: Xenopus Eleutheroembryonic Thyroid Assay
The Xenopus Eleutheroembryonic Thyroid Assay (XETA) provides a short-term screening assay to measure the response of transgenic South African clawed frog (Xenopus laevis) eleutheroembryos to potential thyroid active chemicals. Due to the capacity of a chemical to activate or inhibit the transcription of the genetic construct (THb/ZIP-GFP X.laevis), chemicals can be classified as potentially thyroid active or inactive by quantifying the resulting fluorescence. The test takes into account the entire physiological pathway leading to disruptive outcome and is sensitive to the mode of action.
Test organisms
The South African clawed frog Xenopus laevis is a relevant amphibian model for both, early development and thyroid hormones dependent metamorphosis; and is currently used in two OECD test guidelines: the AMA (amphibian metamorphosis assay, OECD TG 231, OECD 2009) and the LAGDA (larval amphibian growth and development assay, OECD TG 241, OECD 2015).
Due to high genetic homology as well as similar biotransformation systems and homologous endocrine pathways compared to higher vertebrates, test with X. laevis can provide information that might be extrapolated to other taxa.
Test Concentrations
A minimum of three concentration levels, a test medium control (and solvent control, if necessary) should be tested in the absence (“unspiked mode”) and presence (“spiked mode”) of the plasma concentration of thyroid hormone triiodothyronine (T3; 3.25 µg/L). Furthermore, a T3 control and a T4 control (thyroxine; saturation control) are tested. A spacing factor of 3.2 to 10-fold is recommended.
One test comprises three independent, valid runs. Each run includes two replicates per treatment groups and controls. The maximum concentration tested is the limit of solubility, 100 mg/L or the concentration inducing malformations in less than 10% of eleutheroembryos, whichever is lowest.
Course of the test
The transgenic X. laevis embryos are obtained from our partner Laboratoire Watchfrog as early as possible in the development and are reared in-house at 21 ± 1 °C without illumination. The test will be conducted according to the new OECD Test Guideline 248 (OECD 2019).
For the duration of the test, eleutheroembryos from a single spawn are exposed from stage NF 45 (approx. 7 days post fertilisation) to stage NF47 (72 hours) in inert 6-well culture plates under semi-static conditions. No feeding occurs during the test.
Assessments and Endpoints
During the test, embryos are checked for abnormal appearance and death after 24, 48 and 72 hours.
At the end of the test, the normally developed eleutheroembryos are assessed for fluorescence using a spectrofluorometer. Data from the three independent runs are pooled and statistically analysed. If the change in fluorescence compared to the test medium control (“unspiked mode”) or the T3 control (“spiked mode”) is greater than 12 %, the test is considered positive.
Guidelines and Literature
- Niewkoop, P. D., Faber, J. (1994). Normal table of Xenopus laevis (Daudin). Garland Publishing Inc, New York ISBN 0-8153-1896-0
- OECD (2019). Test No. 248: Xenopus Eleutheroembryonic Thyroid Assay (XETA), OECD Giudelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris.