- Your Product Type
- Your Study Type
- Aquatic Ecotoxicology
- Aquatic Invertebrates
- OECD 202: Daphnia sp., Acute Immobilisation Test
- OECD 211: Daphnia magna Reproduction Test
- OECD 235: Chironomus sp., Acute Immobilisation Test
- OECD 218/219: Sediment-Water Chironomid Toxicity Test Using Spiked Sediment/Spiked Water
- OECD 233: Sediment-Water Chironomid Life-Cycle Toxicity Test Using Spiked Water or Spiked Sediment
- OECD 225: Sediment-water Lumbriculus Toxicity Test Using Spiked Sediment
- OECD 242: Potamopyrgus antipodarum Reproduction Test
- OECD 243: Lymnaea stagnalis Reproduction Test
- Fish and other vertebrates
- OECD 203: Fish, Acute Toxicity Test
- OECD 215: Fish Juvenile Growth Study
- OECD 212: Fish, Short-term Toxicity Test on Embryo and Sac-fry Stages
- OECD 231: The Amphibian Metamorphosis Assay
- OECD 236: Fish Embryo Acute Toxicity Test
- OECD 210: Fish, Early-life Stage Toxicity Test
- OECD 229 Fish Short Term Reproduction Assay and OECD 230 21-day Fish Assay
- OECD 240 Medaka Extended One Generation Reproduction Test (MEOGRT)
- OECD 248: Xenopus Eleutheroembryonic Thyroid Assay
- OPPTS 850.1500: Fish Life Cycle Toxicity Test
- OÈCD 234 Fish sexual development test
- Aquatic plants
- Analytical Dose Verification
- Aquatic Invertebrates
- Chemistry
- Biodegradation Studies
- Analytical Chemistry Studies and Residues
- Physical-Chemical Properties Studies
- Storage Stability Studies
- OPPTS 830.6302, OPPTS 830.6303,and OPPTS 830.6304: Physical State, Colour and Odor at 20 °C and at 101.3 kPa
- EU A.1: Melting temperature/range
- EU A.2: Boiling temperature
- EU A.3: Relative density (liquids and solids)
- EU A.4: Vapour pressure
- EU A.5: Surface tension
- EU A.9: Flashpoint
- EU A.10: Flammability (solids)
- EU A.12: Flammability (contact with water)
- EU A.13: Pyrophoric properties of solids and liquids
- EU A.16: Relative self-ignition temperature for solids
- EU A.17: Oxidising properties
- OECD 114: Viscosity of Liquids
- Environmental Fate
- Transformation in Soil
- Transformation in Water
- Transformation in Manure
- Adsorption on Soil and Sewage Sludge
- Bioaccumulation and Bioconcentration
- Terrestrial Ecotoxicology
- Non-target Arthropods
- Non-target arthropod testing with the parasitic wasp (Aphidius rhopalosiphi)
- Non-target arthropod testing with the lacewing (Chrysoperla carnea)
- Non-target arthropod testing with the ladybird beetle (Coccinella septempunctata)
- Non-target arthropod testing with the predatory bug (Orius laevigatus)
- Non-target arthropod testing with the predatory mite (Typhlodromus pyri)
- Non-target arthropod testing with the rove beetle (Aleochara bilineata)
- Non-target arthropod testing with the carabid beetle (Poecilus cupreus)
- Non-target arthropod testing with the wolf spider (Pardosa spec.)
- Soil Organisms
- Honey Bees and other Pollinators
- OECD 213/214: Honey bees, Acute Oral and Acute Contact Toxicity Test
- OECD 245: Honey Bee (Apis Mellifera L.), Chronic Oral Toxicity Test (10-Day Feeding)
- OECD 237: Honey Bee Larval Toxicity Test, Single Exposure
- OECD 239: Honey Bee Larval Toxicity Test
- EPPO 170: Honey Bee Field Study – do plant protection products effect honey bee colonies?
- Oomen et al. 1992: Honey Bee Brood Feeding Study
- OECD 75: Honey Bee Brood Test under Semi-field Conditions in Tunnels
- OECD 246/247 Acute Oral and Contact Toxicity to the Bumblebee, Bombus terrestris L.
- Solitary Bee Acute Contact Toxicity Study in the Laboratory (Osmia sp.) Solitary Bee Acute Oral Toxicity Study in the Laboratory (Osmia sp.) (protocols for ringtests with solitary bees recommended by the non-Apis working group)
- SANTE/11956/2016 rev.9 Residue trials for MRL setting in honey
- Non-target plants
- OECD 208: Terrestrial Plant Test - Seedling Emergence and Seedling Growth Test
- OECD 227: Terrestrial Plant Test - Vegetative Vigour Test
- OCSPP 850.4100: Seedling Emergence and Seedling Growth
- OCSPP 850.4150: Vegetative Vigor
- EPPO PP 1/207(2): Efficacy evaluation of plant protection products, Effects on succeeding crops
- Field Studies
- Non-target Arthropods
- Ecological Modelling
- Quality Assurance
- Testing of Potential Endocrine Disruptors
- Aquatic Ecotoxicology
- News
- Company
- Career
- Contact
OECD 239: Honey Bee Larval Toxicity Test
The purpose of this test design is to determine the chronic toxicity of a plant protection product or chemical to honey bee larvae (Apis mellifera) after a repeated exposure until hatching of the adult bees.
Study Design

Test organisms
The larvae of honey bees are collected from adequately fed and healthy colonies originated from the ibacon apiary to guarantee well known history and physiological status. The tests are performed during the egg laying period of the queen. Three days before grafting the queens of minimum three colonies, each representing a replicate, are confined in their own colonies in an exclusion cage containing an empty comb. The exclusion cage is placed close to combs containing brood. The time of the isolated queens should be held as short as possible in order to minimise the variability in size and age between larvae. After release of the queens the combs containing the eggs are left in the cages inside the hives until hatching (D1).
Course of the test
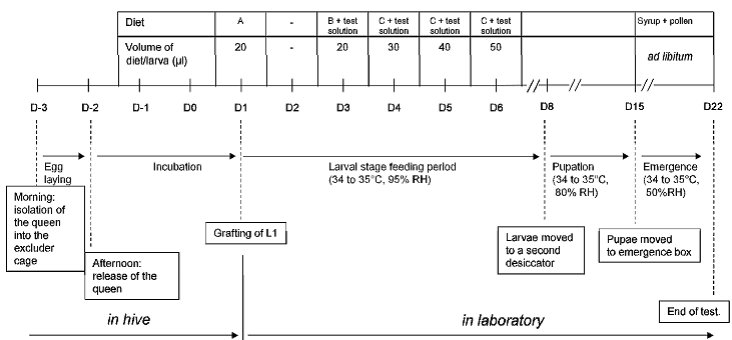
The usual test duration is 25 days in total: D-3 to D1: in hive; D1 to D3: pre exposure; D3 to D6: exposure phase; D7 to D22: pre-pupal, pupal and hatching phase. During the lab phase the larvae are fed with special diets prepared from royal jelly and aqueous solutions of yeast extract glucose and fructose. Diet A, B and C are composed according to the needs of the larvae at different stages of development. Find a schematic representation of the important steps of the larval toxicity test in the following picture.
On D3, D4, D5 and D6, a minimum of twelve well-fed larvae from each of the three colonies are treated with 20, 30, 40 and 50 µl of the diet containing the test solution or reference item at the suitable concentration.
From D1 to D8 the larvae are kept in plexiglas containers placed in an incubator at 34 – 35 °C. A dish filled with saturated K2SO4-solution is placed in the plexiglas containers in order to keep a relative humidity of ca. 95 %. From D8 to D15, the rel. humidity is reduced to ca. 80%. During the hatching phase (D15 to D22) the larvae are moved into hatching boxes at rel. humidity of ca. 50 %.
At D4, D5, D6, D7, D8, D15 and D22 mortalities are checked and counted. . An immobile larva is noted as dead. Dead larvae and pupae are removed systematically for sanitary reasons. At D22 the number of emerged bees is determined and the test is terminated by freezing the exposure units at ≤ -10°C.
Endpoints
The LD50 (i.e. 120 hours, D8) with 95 % confidence limits of the test item and the adult emergence rate on D22 is estimated with suitable statistical methods, if indicated. Other endpoints like a NOEC / NOED can be determined, if appropriate.
Guidelines and Literature
- OECD 239: Guidance Document on Honey Bee (Apis mellifera) Larval Toxicity Test, Repeated Exposure
